Limiting Reactants MCQs with Answers
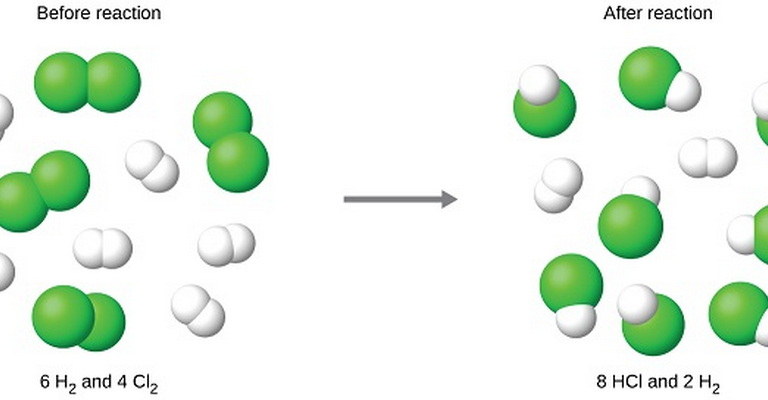
Welcome to the Limiting Reactants MCQs with Answers, it helps learners quickly identify areas for improvement in Limiting Reactants Online Test.
| Limiting reactants are crucial in stoichiometry, the branch of chemistry that deals with the quantitative relationships between reactants and products in chemical reactions. A limiting reactant is the substance that is completely consumed in a reaction, determining the amount of product that can be formed based on its stoichiometric ratio with other reactants.
In a limiting reactants quiz, MCQs on limiting reactants typically assess understanding of how to identify and calculate the amount of product formed using stoichiometric calculations. Stoichiometry multiple choice questions often cover topics such as molar ratios, percent yield, and theoretical yield, which are essential for determining reaction efficiency. Excess reactant MCQs focus on the reactant that remains after a reaction is complete, exploring its role in determining the overall reaction yield. Reaction yield exam questions challenge students to apply their knowledge of limiting reactants and stoichiometry principles to predict the amount of product obtained in real-world scenarios. Chemical equations MCQs delve into balancing chemical equations and understanding how stoichiometric relationships between reactants and products are established. |
Limiting Reactants Online Quiz
By presenting 3 options to choose from, Limiting Reactants Quiz which cover a wide range of topics and levels of difficulty, making them adaptable to various learning objectives and preferences. You will have to read all the given answers of Limiting Reactants Questions and Answers and click over the correct answer.
- Test Name: Limiting Reactants MCQ Quiz Practice
- Type: Quiz Test
- Total Questions: 40
- Total Marks: 40
- Time: 40 minutes
Note: Answer of the questions will change randomly each time you start the test. Practice each quiz test at least 3 times if you want to secure High Marks. Once you are finished, click the View Results button. If any answer looks wrong to you in Quiz, simply click on question and comment below that question, so that we can update the answer in the quiz section.
Download Certificate of Limiting Reactants Test
On the end of Quiz, you can download the certificate of the quiz if you got more than 70% marks.
Limiting Reactants Flashcards
In the reaction 2H₂ + O₂ → 2H₂O, if you have 4 moles of H₂ and 2 moles of O₂, what is the limiting reactant?
H₂
In the reaction N₂ + 3H₂ → 2NH₃, if you have 1 mole of N₂ and 3 moles of H₂, what is the limiting reactant?
H₂
In the reaction 2SO₂ + O₂ → 2SO₃, if you have 5 moles of SO₂ and 2 moles of O₂, what is the limiting reactant?
SO₂
In the reaction 4Fe + 3O₂ → 2Fe₂O₃, if you have 8 moles of Fe and 6 moles of O₂, what is the limiting reactant?
Fe
In the reaction 2Na + Cl₂ → 2NaCl, if you have 4 moles of Na and 1 mole of Cl₂, what is the limiting reactant?
Cl₂
In the reaction 2C + O₂ → 2CO, if you have 3 moles of C and 2 moles of O₂, what is the limiting reactant?
C
In the reaction 3H₂ + N₂ → 2NH₃, if you have 3 moles of H₂ and 1 mole of N₂, what is the limiting reactant?
H₂
In the reaction 2Mg + O₂ → 2MgO, if you have 4 moles of Mg and 3 moles of O₂, what is the limiting reactant?
Mg
In the reaction 2Al + 3Cl₂ → 2AlCl₃, if you have 5 moles of Al and 5 moles of Cl₂, what is the limiting reactant?
Cl₂
In the reaction 2H₂ + O₂ → 2H₂O, if you have 5 moles of H₂ and 2 moles of O₂, what is the limiting reactant?
O₂
In the reaction C + O₂ → CO₂, if you have 2 moles of C and 1 mole of O₂, what is the limiting reactant?
O₂
In the reaction 2K + Cl₂ → 2KCl, if you have 3 moles of K and 2 moles of Cl₂, what is the limiting reactant?
K
In the reaction 2H₂ + O₂ → 2H₂O, if you have 6 moles of H₂ and 3 moles of O₂, what is the limiting reactant?
None
In the reaction 2Fe + 3Cl₂ → 2FeCl₃, if you have 4 moles of Fe and 6 moles of Cl₂, what is the limiting reactant?
Fe
In the reaction 3H₂ + N₂ → 2NH₃, if you have 6 moles of H₂ and 2 moles of N₂, what is the limiting reactant?
None
In the reaction 2Al + 3Cl₂ → 2AlCl₃, if you have 2 moles of Al and 6 moles of Cl₂, what is the limiting reactant?
Al
In the reaction 2C + O₂ → 2CO, if you have 4 moles of C and 1 mole of O₂, what is the limiting reactant?
O₂
In the reaction 2Na + Cl₂ → 2NaCl, if you have 6 moles of Na and 2 moles of Cl₂, what is the limiting reactant?
None
In the reaction N₂ + 3H₂ → 2NH₃, if you have 2 moles of N₂ and 6 moles of H₂, what is the limiting reactant?
None
In the reaction 4Fe + 3O₂ → 2Fe₂O₃, if you have 6 moles of Fe and 3 moles of O₂, what is the limiting reactant?
Fe
In the reaction 2H₂ + O₂ → 2H₂O, if you have 2 moles of H₂ and 2 moles of O₂, what is the limiting reactant?
H₂
In the reaction 2C + O₂ → 2CO, if you have 4 moles of C and 2 moles of O₂, what is the limiting reactant?
C
In the reaction 2Mg + O₂ → 2MgO, if you have 4 moles of Mg and 2 moles of O₂, what is the limiting reactant?
None
In the reaction 2Al + 3Cl₂ → 2AlCl₃, if you have 3 moles of Al and 3 moles of Cl₂, what is the limiting reactant?
Cl₂
In the reaction N₂ + 3H₂ → 2NH₃, if you have 2 moles of N₂ and 8 moles of H₂, what is the limiting reactant?
N₂
In the reaction 4Fe + 3O₂ → 2Fe₂O₃, if you have 8 moles of Fe and 4 moles of O₂, what is the limiting reactant?
Fe
In the reaction 2K + Cl₂ → 2KCl, if you have 2 moles of K and 2 moles of Cl₂, what is the limiting reactant?
K
In the reaction 2H₂ + O₂ → 2H₂O, if you have 2 moles of H₂ and 1 mole of O₂, what is the limiting reactant?
H₂
In the reaction 2Fe + 3Cl₂ → 2FeCl₃, if you have 4 moles of Fe and 4 moles of Cl₂, what is the limiting reactant?
Cl₂
In the reaction 3H₂ + N₂ → 2NH₃, if you have 2 moles of H₂ and 1 mole of N₂, what is the limiting reactant?
H₂
In the reaction 2Al + 3Cl₂ → 2AlCl₃, if you have 6 moles of Al and 9 moles of Cl₂, what is the limiting reactant?
None
In the reaction 2C + O₂ → 2CO, if you have 1 mole of C and 1 mole of O₂, what is the limiting reactant?
C
In the reaction 2Na + Cl₂ → 2NaCl, if you have 2 moles of Na and 4 moles of Cl₂, what is the limiting reactant?
Na
In the reaction N₂ + 3H₂ → 2NH₃, if you have 4 moles of N₂ and 12 moles of H₂, what is the limiting reactant?
None
In the reaction 4Fe + 3O₂ → 2Fe₂O₃, if you have 4 moles of Fe and 4 moles of O₂, what is the limiting reactant?
Fe
In the reaction 2H₂ + O₂ → 2H₂O, if you have 1 mole of H₂ and 1 mole of O₂, what is the limiting reactant?
H₂
In the reaction 2K + Cl₂ → 2KCl, if you have 1 mole of K and 2 moles of Cl₂, what is the limiting reactant?
K
In the reaction N₂ + 3H₂ → 2NH₃, if you have 5 moles of N₂ and 12 moles of H₂, what is the limiting reactant?
H₂
If you are interested to enhance your knowledge regarding Physics, Computer, and Biology please click on the link of each category, you will be redirected to dedicated website for each category.




