Balancing Equations MCQs with Answers

Welcome to the Balancing Equations MCQs with Answers, it helps learners quickly identify areas for improvement in Balancing Equations Online Test.
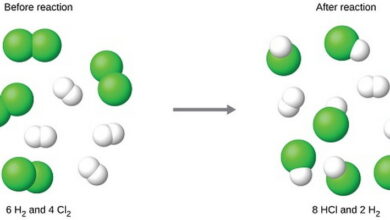
| Balancing equations is a fundamental skill in chemistry that involves ensuring that the number of atoms of each element is the same on both sides of a chemical equation. This process maintains the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction.
In a balancing equations quiz, MCQs on balancing equations typically assess students’ proficiency in balancing complex chemical reactions. These questions may include scenarios involving different types of reactions, such as synthesis, decomposition, combustion, and redox reactions. Chemical equation multiple choice questions explore various aspects of writing and interpreting chemical equations, including identifying reactants and products, understanding the stoichiometry involved, and predicting the outcomes of reactions based on balanced equations. Stoichiometry balancing MCQs focus on applying balancing principles to stoichiometric calculations, ensuring accuracy in determining reactant and product quantities in chemical reactions. Reaction balancing exam questions challenge students to apply their understanding of balancing equations to practical situations, such as determining reaction yields or identifying limiting reactants. Redox reactions MCQs delve into balancing oxidation-reduction reactions, emphasizing the transfer of electrons between reactants and products. |
Balancing Equations Online Quiz
By presenting 3 options to choose from, Balancing Equations Quiz which cover a wide range of topics and levels of difficulty, making them adaptable to various learning objectives and preferences. You will have to read all the given answers of Balancing Equations Questions and Answers and click over the correct answer.
- Test Name: Balancing Equations MCQ Quiz Practice
- Type: Quiz Test
- Total Questions: 40
- Total Marks: 40
- Time: 40 minutes
Note: Answer of the questions will change randomly each time you start the test. Practice each quiz test at least 3 times if you want to secure High Marks. Once you are finished, click the View Results button. If any answer looks wrong to you in Quiz, simply click on question and comment below that question, so that we can update the answer in the quiz section.
Download Certificate of Balancing Equations Test
On the end of Quiz, you can download the certificate of the quiz if you got more than 70% marks.
Balancing Equations Flashcards
What is the balanced equation for the reaction: Hydrogen gas reacts with oxygen gas to form water?
2H₂ + O₂ → 2H₂O
What is the balanced equation for the reaction: Sodium reacts with chlorine gas to form sodium chloride?
2Na + Cl₂ → 2NaCl
What is the balanced equation for the reaction: Magnesium reacts with hydrochloric acid to form magnesium chloride and hydrogen gas?
Mg + 2HCl → MgCl₂ + H₂
What is the balanced equation for the reaction: Carbon dioxide gas reacts with water to form carbonic acid?
CO₂ + H₂O → H₂CO₃
What is the balanced equation for the reaction: Sulfuric acid reacts with potassium hydroxide to form potassium sulfate and water?
H₂SO₄ + 2KOH → K₂SO₄ + 2H₂O
What is the balanced equation for the reaction: Iron reacts with sulfur to form iron(II) sulfide?
Fe + S → FeS
What is the balanced equation for the reaction: Nitric acid reacts with calcium hydroxide to form calcium nitrate and water?
2HNO₃ + Ca(OH)₂ → Ca(NO₃)₂ + 2H₂O
What is the balanced equation for the reaction: Ammonia reacts with oxygen gas to form nitrogen dioxide and water?
4NH₃ + 7O₂ → 4NO₂ + 6H₂O
What is the balanced equation for the reaction: Hydrochloric acid reacts with sodium hydroxide to form sodium chloride and water?
HCl + NaOH → NaCl + H₂O
What is the balanced equation for the reaction: Potassium reacts with water to form potassium hydroxide and hydrogen gas?
2K + 2H₂O → 2KOH + H₂
What is the balanced equation for the reaction: Nitrogen gas reacts with hydrogen gas to form ammonia?
N₂ + 3H₂ → 2NH₃
What is the balanced equation for the reaction: Aluminum reacts with oxygen gas to form aluminum oxide?
4Al + 3O₂ → 2Al₂O₃
What is the balanced equation for the reaction: Sulfur dioxide gas reacts with oxygen gas to form sulfur trioxide gas?
2SO₂ + O₂ → 2SO₃
What is the balanced equation for the reaction: Nitrogen gas reacts with oxygen gas to form nitrogen dioxide?
2NO + O₂ → 2NO₂
What is the balanced equation for the reaction: Calcium reacts with sulfuric acid to form calcium sulfate and hydrogen gas?
Ca + H₂SO₄ → CaSO₄ + H₂
What is the balanced equation for the reaction: Sodium carbonate reacts with hydrochloric acid to form sodium chloride, water, and carbon dioxide gas?
Na₂CO₃ + 2HCl → 2NaCl + H₂O + CO₂
What is the balanced equation for the reaction: Ethanol reacts with oxygen gas to form carbon dioxide and water?
C₂H₅OH + 3O₂ → 2CO₂ + 3H₂O
What is the balanced equation for the reaction: Hydrogen peroxide decomposes into water and oxygen gas?
2H₂O₂ → 2H₂O + O₂
What is the balanced equation for the reaction: Potassium chlorate decomposes into potassium chloride and oxygen gas when heated?
2KClO₃ → 2KCl + 3O₂
What is the balanced equation for the reaction: Sodium bicarbonate decomposes into sodium carbonate, water, and carbon dioxide when heated?
2NaHCO₃ → Na₂CO₃ + H₂O + CO₂
What is the balanced equation for the reaction: Phosphorus reacts with chlorine gas to form phosphorus pentachloride?
P₄ + 10Cl₂ → 4PCl₅
What is the balanced equation for the reaction: Zinc reacts with hydrochloric acid to form zinc chloride and hydrogen gas?
Zn + 2HCl → ZnCl₂ + H₂
What is the balanced equation for the reaction: Carbon monoxide reacts with oxygen gas to form carbon dioxide?
2CO + O₂ → 2CO₂
What is the balanced equation for the reaction: Potassium reacts with chlorine gas to form potassium chloride?
2K + Cl₂ → 2KCl
What is the balanced equation for the reaction: Calcium carbonate decomposes into calcium oxide and carbon dioxide when heated?
CaCO₃ → CaO + CO₂
What is the balanced equation for the reaction: Magnesium oxide reacts with hydrochloric acid to form magnesium chloride and water?
MgO + 2HCl → MgCl₂ + H₂O
What is the balanced equation for the reaction: Hydrogen gas reacts with iodine gas to form hydrogen iodide?
H₂ + I₂ → 2HI
What is the balanced equation for the reaction: Aluminum reacts with sulfuric acid to form aluminum sulfate and hydrogen gas?
2Al + 3H₂SO₄ → Al₂(SO₄)₃ + 3H₂
What is the balanced equation for the reaction: Barium hydroxide reacts with hydrochloric acid to form barium chloride and water?
Ba(OH)₂ + 2HCl → BaCl₂ + 2H₂O
What is the balanced equation for the reaction: Sodium reacts with water to form sodium hydroxide and hydrogen gas?
2Na + 2H₂O → 2NaOH + H₂
What is the balanced equation for the reaction: Calcium reacts with nitrogen gas to form calcium nitride?
3Ca + N₂ → Ca₃N₂
What is the balanced equation for the reaction: Methane reacts with chlorine gas to form carbon tetrachloride and hydrogen chloride?
CH₄ + 4Cl₂ → CCl₄ + 4HCl
What is the balanced equation for the reaction: Calcium oxide reacts with carbon dioxide to form calcium carbonate?
CaO + CO₂ → CaCO₃
What is the balanced equation for the reaction: Sulfuric acid reacts with sodium hydroxide to form sodium sulfate and water?
H₂SO₄ + 2NaOH → Na₂SO₄ + 2H₂O
What is the balanced equation for the reaction: Iron(III) oxide reacts with carbon monoxide to form iron and carbon dioxide?
Fe₂O₃ + 3CO → 2Fe + 3CO₂
What is the balanced equation for the reaction: Phosphoric acid reacts with sodium hydroxide to form sodium phosphate and water?
H₃PO₄ + 3NaOH → Na₃PO₄ + 3H₂O
What is the balanced equation for the reaction: Potassium hydroxide reacts with carbon dioxide to form potassium carbonate and water?
2KOH + CO₂ → K₂CO₃ + H₂O
What is the balanced equation for the reaction: Aluminum reacts with bromine gas to form aluminum bromide?
2Al + 3Br₂ → 2AlBr₃
If you are interested to enhance your knowledge regarding Physics, Computer, and Biology please click on the link of each category, you will be redirected to dedicated website for each category.