Chemistry Electrochemistry MCQs with Answers

Welcome to the Chemistry Electrochemistry MCQs with Answers, it helps learners quickly identify areas for improvement in Chemistry Electrochemistry Online Test.
| Electrochemistry is a branch of chemistry that studies the relationship between chemical reactions and electricity. It explores how chemical reactions can be driven by applying electrical energy or how electrical energy can be generated from spontaneous chemical reactions. This field is pivotal in understanding batteries, corrosion processes, electrolysis, and electroplating.
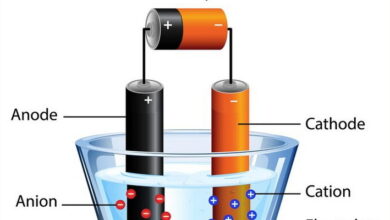
In an electrochemistry quiz, MCQs on electrochemistry typically cover fundamental concepts and applications. Redox reactions multiple choice questions focus on oxidation-reduction processes, including identifying oxidizing and reducing agents, balancing redox equations, and predicting reaction spontaneity. Electrochemical cells MCQs delve into different types of electrochemical cells, such as galvanic cells (voltaic cells) and electrolytic cells, emphasizing their construction, operation principles, and applications. Electrode potentials exam questions explore the measurement and significance of electrode potentials in predicting the direction and feasibility of redox reactions. This includes understanding standard electrode potentials and their relation to cell potentials in electrochemical cells. Galvanic cells MCQs examine the factors affecting cell potential, including concentration gradients, temperature, and electrode materials. |
Chemistry Electrochemistry Online Quiz
By presenting 3 options to choose from, Chemistry Electrochemistry Quiz which cover a wide range of topics and levels of difficulty, making them adaptable to various learning objectives and preferences. You will have to read all the given answers of Chemistry Electrochemistry Questions and Answers and click over the correct answer.
- Test Name: Chemistry Electrochemistry MCQ Quiz Practice
- Type: Quiz Test
- Total Questions: 40
- Total Marks: 40
- Time: 40 minutes
Note: Answer of the questions will change randomly each time you start the test. Practice each quiz test at least 3 times if you want to secure High Marks. Once you are finished, click the View Results button. If any answer looks wrong to you in Quiz, simply click on question and comment below that question, so that we can update the answer in the quiz section.
Download Certificate of Chemistry Electrochemistry Test
On the end of Quiz, you can download the certificate of the quiz if you got more than 70% marks.
Chemistry Electrochemistry Flashcards
Which law states that the product of the masses of the ions liberated at the electrodes during electrolysis is directly proportional to their chemical equivalent weights?
Faraday's second law
The process of converting a substance from its ionic form to its metallic form is called ____________.
Reduction
The device used to measure the potential difference between two electrodes is called a __________.
Voltmeter
The movement of ions in a solution under the influence of an electric field is called __________.
Electrolysis
Faraday's first law of electrolysis states that the mass of the substance deposited or liberated at an electrode during electrolysis is directly proportional to the __________.
Current
The process of depositing a layer of one metal over another by means of electrolysis is called ____________.
Electroplating
The process of conversion of chemical energy into electrical energy is called ________.
Electrolysis
What is the electrode potential of a half-cell when the concentration of its ions is 1 M and the pressure of gases is 1 atm?
Standard electrode potential
What is the potential difference required to drive a non-spontaneous reaction in an electrochemical cell?
Electrolysis potential
What is the potential difference required to reduce a species at the cathode of an electrochemical cell?
Reduction potential
What is the potential difference required to oxidize a species at the anode of an electrochemical cell?
Oxidation potential
What is the process of coating a metal object with a thin layer of zinc to prevent corrosion?
Galvanization
What is the process of using an external source of electricity to drive a non-spontaneous chemical reaction?
Electrolysis
What is the process of depositing a thin layer of metal onto an object using electrolysis?
Electroplating
What is the movement of ions in an electrolyte solution under the influence of an electric field?
Electrolysis
If you are interested to enhance your knowledge regarding Physics, Computer, and Biology please click on the link of each category, you will be redirected to dedicated website for each category.