Electrochemistry MCQs with Answers

Welcome to the Electrochemistry MCQs with Answers, it helps learners quickly identify areas for improvement in Electrochemistry Online Test.
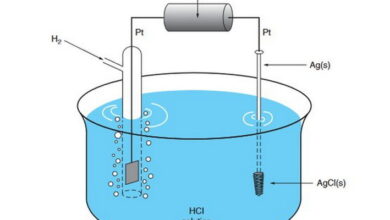
| Electrochemistry is a branch of chemistry that deals with the study of chemical reactions involving the transfer of electrons between reactants, and the relationship between chemical reactions and electricity. It encompasses both spontaneous processes, such as those occurring in galvanic cells (voltaic cells), and non-spontaneous processes, such as those used in electrolysis.
In an electrochemistry quiz, MCQs on electrochemistry cover various aspects of this field. Redox reactions multiple choice questions focus on oxidation-reduction processes, including identifying oxidizing and reducing agents, balancing redox equations, and predicting the feasibility of reactions based on standard electrode potentials. Electrochemical cells MCQs delve into different types of electrochemical cells, their components, and their applications in generating electrical energy or driving chemical reactions. This includes understanding the operation principles of galvanic cells and electrolytic cells. Electrode potentials exam questions explore the measurement and significance of electrode potentials in predicting the direction and extent of redox reactions. This involves understanding concepts like standard electrode potentials, cell potentials, and the Nernst equation. Galvanic cells MCQs specifically focus on the factors influencing cell potential, such as concentration gradients, temperature, and electrode materials, and their role in generating electricity. |
Electrochemistry Online Quiz
By presenting 3 options to choose from, Electrochemistry Quiz which cover a wide range of topics and levels of difficulty, making them adaptable to various learning objectives and preferences. You will have to read all the given answers of Electrochemistry Questions and Answers and click over the correct answer.
- Test Name: Electrochemistry MCQ Quiz Practice
- Type: Quiz Test
- Total Questions: 40
- Total Marks: 40
- Time: 40 minutes
Note: Answer of the questions will change randomly each time you start the test. Practice each quiz test at least 3 times if you want to secure High Marks. Once you are finished, click the View Results button. If any answer looks wrong to you in Quiz, simply click on question and comment below that question, so that we can update the answer in the quiz section.
Download Certificate of Electrochemistry Test
On the end of Quiz, you can download the certificate of the quiz if you got more than 70% marks.
Electrochemistry Flashcards
What is the potential difference between two half-cells in an electrochemical cell called?
Cell potential
Which law states that the current flowing through a conductor is directly proportional to the voltage across it?
Ohm's law
What is the term for the movement of ions in solution under the influence of an electric field?
Electrolysis
What is the process of depositing a thin layer of metal onto a surface using electricity?
Electroplating
Which of the following is a measure of a material's opposition to the flow of electric current?
Resistance
What is the process of using an external source of electric current to force a non-spontaneous chemical reaction?
Electrolysis
What is the process of preventing corrosion of a metal by applying a protective layer of another metal?
Galvanization
What is the term for the electrode potential of a half-cell measured against a standard hydrogen electrode?
Standard electrode potential
What is the formula for calculating cell potential under standard conditions?
E°cell = E°cathode - E°anode
What is the electromotive force (EMF) of a cell under standard conditions called?
Standard cell potential
What is the term for the maximum potential difference between the cathode and anode in an electrochemical cell?
Cell potential
What is the process of using electricity to coat a metal object with a thin layer of metal called?
Electroplating
What is the term for the reduction potential of an electrode measured against a standard hydrogen electrode?
Standard reduction potential
Which of the following metals is commonly used as a reference electrode in electrochemistry?
Platinum
What is the term for the ratio of the actual voltage of a cell to its standard cell potential?
Cell efficiency
If you are interested to enhance your knowledge regarding Physics, Computer, and Biology please click on the link of each category, you will be redirected to dedicated website for each category.