Reaction Mechanisms MCQs with Answers
Welcome to the Reaction Mechanisms MCQs with Answers, it helps learners quickly identify areas for improvement in Reaction Mechanisms Online Test.
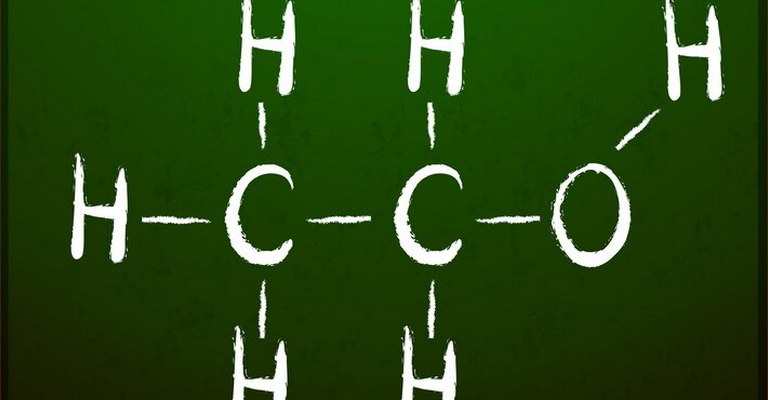
| Reaction mechanisms are crucial in understanding how chemical reactions occur at a molecular level. They detail the step-by-step process by which reactants transform into products, including the formation of intermediates. Studying reaction mechanisms is fundamental in organic chemistry, as it provides insights into the behavior and reactivity of various compounds.
For those preparing for exams or assessments, Reaction Mechanisms MCQs are an excellent way to test comprehension. Organic Chemistry Reaction Mechanisms MCQs focus specifically on the mechanisms prevalent in organic reactions, such as nucleophilic substitutions, electrophilic additions, and free radical reactions. These multiple choice questions help in identifying key steps and intermediates involved in reactions. Chemical Reaction Mechanisms Multiple Choice Questions often include topics like transition states, reaction coordinates, and energy profiles. These questions are designed to challenge and enhance understanding of complex reaction pathways. Taking Reaction Mechanisms Quiz Questions can significantly aid in mastering the subject, as they cover a broad spectrum of mechanisms and their intermediates. Advanced Reaction Mechanisms Questions delve deeper into sophisticated topics, requiring a more profound knowledge of the principles and applications of reaction mechanisms. MCQs on Reaction Mechanisms and Intermediates test the ability to predict products, understand the kinetics of reactions, and recognize various reaction intermediates, ensuring a comprehensive grasp of the subject. |
Reaction Mechanisms Online Quiz
By presenting 3 options to choose from, Reaction Mechanisms Quiz which cover a wide range of topics and levels of difficulty, making them adaptable to various learning objectives and preferences. You will have to read all the given answers of Reaction Mechanisms Questions and Answers and click over the correct answer.
- Test Name: Reaction Mechanisms MCQ Quiz Practice
- Type: Quiz Test
- Total Questions: 40
- Total Marks: 40
- Time: 40 minutes
Note: Answer of the questions will change randomly each time you start the test. Practice each quiz test at least 3 times if you want to secure High Marks. Once you are finished, click the View Results button. If any answer looks wrong to you in Quiz, simply click on question and comment below that question, so that we can update the answer in the quiz section.
Download Certificate of Reaction Mechanisms Test
On the end of Quiz, you can download the certificate of the quiz if you got more than 70% marks.
Reaction Mechanisms Flashcards
What is the term for the series of steps that describe the pathway from reactants to products?
Reaction mechanism
Which term describes a substance that increases the rate of a reaction without being consumed?
Catalyst
What is a reaction intermediate?
Species formed during the reaction that does not appear in the overall equation
In an E2 elimination reaction, how many molecules are involved in the transition state?
Two molecules
What happens during the propagation step of a radical chain reaction?
Radicals react and form new radicals
What is the term for the energy difference between reactants and the transition state?
Activation energy
If you are interested to enhance your knowledge regarding Physics, Computer, and Biology please click on the link of each category, you will be redirected to dedicated website for each category.