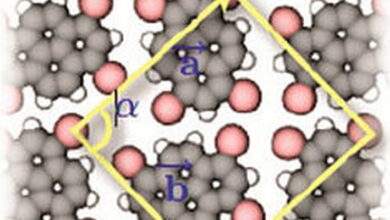
Non-Covalent Interactions MCQs with Answers
Welcome to the Non-Covalent Interactions MCQs with Answers, it helps learners quickly identify areas for improvement in Non-Covalent Interactions Online Test.
| Non-covalent interactions are crucial forces that influence the behavior and properties of molecules without forming covalent bonds. These interactions include hydrogen bonding, van der Waals forces, ionic interactions, and hydrophobic effects. They play a significant role in biological systems, supramolecular chemistry, and materials science.
In a non-covalent interactions quiz, MCQs on non-covalent interactions typically cover the fundamental principles and types of these interactions. Hydrogen bonding multiple choice questions explore the nature, strength, and significance of hydrogen bonds in stabilizing molecular structures like DNA and proteins. Van der Waals forces MCQs focus on the weak, short-range forces arising from induced dipoles, crucial for understanding the physical properties of gases, liquids, and solids. Non-covalent bonding exam questions delve into the thermodynamics and kinetics of these interactions, including concepts like binding energy and molecular recognition. Intermolecular forces MCQs examine the collective impact of various non-covalent interactions on the physical and chemical properties of substances, such as boiling points, solubility, and mechanical strength. |
Non-Covalent Interactions Online Quiz
By presenting 3 options to choose from, Non-Covalent Interactions Quiz which cover a wide range of topics and levels of difficulty, making them adaptable to various learning objectives and preferences. You will have to read all the given answers of Non-Covalent Interactions Questions and Answers and click over the correct answer.
- Test Name: Non-Covalent Interactions MCQ Quiz Practice
- Type: Quiz Test
- Total Questions: 40
- Total Marks: 40
- Time: 40 minutes
Note: Answer of the questions will change randomly each time you start the test. Practice each quiz test at least 3 times if you want to secure High Marks. Once you are finished, click the View Results button. If any answer looks wrong to you in Quiz, simply click on question and comment below that question, so that we can update the answer in the quiz section.
Download Certificate of Non-Covalent Interactions Test
On the end of Quiz, you can download the certificate of the quiz if you got more than 70% marks.
Non-Covalent Interactions Flashcards
Which type of non-covalent interaction involves the attraction between a hydrogen atom and an electronegative atom?
Hydrogen Bonding
What is the term for the weak attraction between temporary dipoles in non-polar molecules?
London Dispersion Forces
Which type of non-covalent interaction involves the attraction between permanent dipoles in polar molecules?
Dipole-Dipole Interaction
What is the term for the attraction between an ion with a full positive charge and an ion with a full negative charge?
Ionic Interaction
Which type of non-covalent interaction involves the repulsion between electron clouds of adjacent molecules?
Steric Hindrance
Which type of non-covalent interaction involves the attraction between a metal ion and a molecule with lone pair electrons?
Coordination Interaction
What is the term for the weak interaction between a π-electron system and a nearby atom or molecule?
π-π Stacking
Which type of non-covalent interaction involves the alignment of molecules in response to an external electric field?
Induced Dipole Moment
What is the term for the weak interaction between a hydrogen atom bonded to an electronegative atom and a nearby lone pair?
Hydrogen Bonding
Which type of non-covalent interaction involves the attraction between a charged group on a molecule and an opposite charge nearby?
Electrostatic Interaction
Which type of non-covalent interaction involves the interaction between adjacent electron clouds in molecules?
Van der Waals Forces
What is the term for the interaction between a metal ion and a molecule with multiple donor atoms?
Coordination Interaction
Which type of non-covalent interaction involves the stacking of aromatic rings in adjacent molecules?
π-π Stacking
What is the term for the weak interaction between a permanent dipole in one molecule and an induced dipole in another molecule?
Dipole-Induced Dipole Interaction
Which type of non-covalent interaction involves the interaction between a metal ion and a ligand with multiple donor atoms?
Metal-Ligand Coordination
What is the term for the attraction between two molecules due to the presence of a permanent dipole in one molecule and an induced dipole in the other?
Dipole-Induced Dipole Interaction
Which type of non-covalent interaction involves the weak attraction between temporary dipoles in molecules?
London Dispersion Forces
What is the term for the interaction between a metal ion and a ligand with lone pair electrons?
Coordination Interaction
Which type of non-covalent interaction involves the interaction between a metal ion and a ligand with lone pair electrons?
Coordination Interaction
What is the term for the interaction between adjacent electron clouds in molecules?
Van der Waals Forces
Which type of non-covalent interaction involves the interaction between a metal ion and a ligand with multiple donor atoms?
Metal-Ligand Coordination
What is the term for the interaction between a metal ion and a ligand with multiple donor atoms?
Coordination Interaction
Which type of non-covalent interaction involves the stacking of aromatic rings in adjacent molecules?
π-π Stacking
What is the term for the weak interaction between a permanent dipole in one molecule and an induced dipole in another molecule?
Dipole-Induced Dipole Interaction
Which type of non-covalent interaction involves the interaction between a metal ion and a ligand with multiple donor atoms?
Metal-Ligand Coordination
What is the term for the attraction between two molecules due to the presence of a permanent dipole in one molecule and an induced dipole in the other?
Dipole-Induced Dipole Interaction
Which type of non-covalent interaction involves the weak attraction between temporary dipoles in molecules?
London Dispersion Forces
What is the term for the interaction between a metal ion and a ligand with lone pair electrons?
Coordination Interaction
Which type of non-covalent interaction involves the interaction between a metal ion and a ligand with lone pair electrons?
Coordination Interaction
What is the term for the interaction between adjacent electron clouds in molecules?
Van der Waals Forces
Which type of non-covalent interaction involves the attraction between a hydrogen atom and an electronegative atom?
Hydrogen Bonding
What is the term for the weak attraction between temporary dipoles in non-polar molecules?
London Dispersion Forces
Which type of non-covalent interaction involves the attraction between permanent dipoles in polar molecules?
Dipole-Dipole Interaction
What is the term for the attraction between an ion with a full positive charge and an ion with a full negative charge?
Ionic Interaction
Which type of non-covalent interaction involves the repulsion between electron clouds of adjacent molecules?
Steric Hindrance
If you are interested to enhance your knowledge regarding Physics, Computer, and Biology please click on the link of each category, you will be redirected to dedicated website for each category.