Reaction Rates MCQs with Answers
Welcome to the Reaction Rates MCQs with Answers, it helps learners quickly identify areas for improvement in Reaction Rates Online Test.
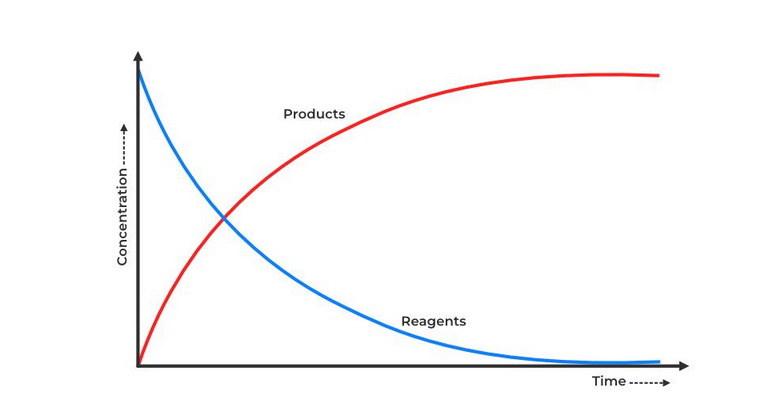
| Reaction rates refer to the speed at which chemical reactions occur and are crucial in understanding reaction kinetics. The rate of a reaction is typically measured as the change in concentration of reactants or products per unit time. Factors influencing reaction rates include the nature of reactants, temperature, concentration, surface area, and the presence of catalysts.
For those studying or working in chemistry, Reaction Rates MCQs with Answers offer an effective way to grasp and apply these concepts. These multiple choice questions cover a range of topics, including how to calculate reaction rates from experimental data, interpret rate laws, and understand the effects of various factors on reaction rates. Multiple Choice Questions on Reaction Rates in quizzes provide scenarios where learners can apply their knowledge to real-world examples. These questions often explore how changes in temperature, concentration, or catalysts affect reaction rates and equilibrium. A Reaction Rates Practice Test is beneficial for consolidating knowledge and identifying areas for improvement before exams. It allows individuals to simulate exam conditions and gauge their readiness. Reaction Rates Exam Questions are designed to assess a deeper understanding of kinetics, requiring application of rate laws, interpretation of experimental results, and critical analysis of reaction mechanisms. Mastering reaction rates is essential for predicting and controlling chemical processes in both academic and industrial settings. |
Reaction Rates Online Quiz
By presenting 3 options to choose from, Reaction Rates Quiz which cover a wide range of topics and levels of difficulty, making them adaptable to various learning objectives and preferences. You will have to read all the given answers of Reaction Rates Questions and Answers and click over the correct answer.
- Test Name: Reaction Rates MCQ Quiz Practice
- Type: Quiz Test
- Total Questions: 40
- Total Marks: 40
- Time: 40 minutes
Note: Answer of the questions will change randomly each time you start the test. Practice each quiz test at least 3 times if you want to secure High Marks. Once you are finished, click the View Results button. If any answer looks wrong to you in Quiz, simply click on question and comment below that question, so that we can update the answer in the quiz section.
Download Certificate of Reaction Rates Test
On the end of Quiz, you can download the certificate of the quiz if you got more than 70% marks.
Reaction Rates Flashcards
What is the definition of reaction rate?
Change in concentration of reactants or products per unit time
What is the term for a substance that increases the rate of a reaction without being consumed?
Catalyst
What is the effect of increasing the surface area of a solid reactant on the reaction rate?
Increases the rate
How does decreasing the particle size of a solid reactant affect the reaction rate?
Increases the rate
What is the term for the energy barrier that must be overcome for a reaction to proceed?
Activation energy
How does the presence of an enzyme affect the reaction rate in biological systems?
Increases the rate
How does the rate of a reaction change with the increase of reactant concentration in a first-order reaction?
Directly proportional
How does the rate of reaction change with time for a reaction that follows first-order kinetics?
Decreases exponentially
In a reaction A → B, if the concentration of A is doubled, how is the rate affected in a first-order reaction?
Doubles
How does increasing the concentration of a product affect the reaction rate?
Generally decreases the rate
What is the effect of increasing the surface area of a catalyst on the reaction rate?
Increases the rate
How does the nature of reactants affect the reaction rate?
Different reactants react at different rates
What is the term for the time taken for the concentration of a reactant to decrease by half?
Half-life
In a zero-order reaction, how does the rate change with the increase in reactant concentration?
Remains constant
What is the effect of pressure on the rate of a reaction involving solids or liquids?
Generally no effect
How does the nature of the solvent affect the reaction rate?
Can either increase or decrease the rate
In a second-order reaction, how does the rate change if the concentration of one reactant is doubled?
Quadruples
How does the concentration of a reactant affect the rate of reaction in a zero-order reaction?
No effect
How does increasing the ionic strength of a solution affect the rate of a reaction?
Increases the rate
How does the addition of a non-reactive gas affect the rate of a reaction involving gases?
No effect
What is the effect of increasing the concentration of a catalyst on the rate of reaction?
Increases the rate
How does a decrease in particle size of a solid catalyst affect the reaction rate?
Increases the rate
How does the presence of impurities affect the rate of a catalytic reaction?
Generally decreases the rate
If you are interested to enhance your knowledge regarding Physics, Computer, and Biology please click on the link of each category, you will be redirected to dedicated website for each category.