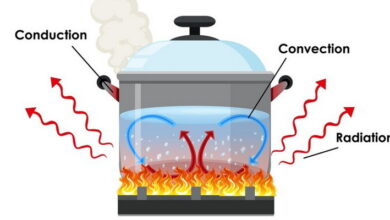
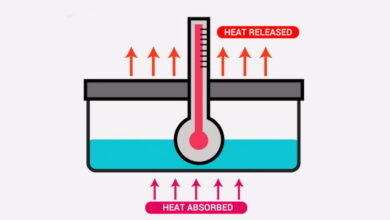
Thermodynamics MCQs with Answers
Welcome to the Thermodynamics MCQs with Answers, it helps learners quickly identify areas for improvement in Thermodynamics Online Test.
| Thermodynamics is the branch of physics that deals with the relationships between heat, work, temperature, and energy. It is a fundamental field in understanding how energy transfers and transformations occur in physical systems. In educational contexts, Thermodynamics MCQs (Multiple Choice Questions), Thermodynamics quiz, and Thermodynamics practice questions are commonly used to assess understanding and knowledge retention.
Thermodynamics MCQs cover a wide range of topics including the Laws of Thermodynamics MCQs, which are foundational principles governing energy exchange. These laws include the zeroth law, stating thermal equilibrium among bodies, the first law, which expresses conservation of energy, the second law, defining the direction of spontaneous processes, and the third law, focusing on absolute zero temperature and entropy. Students engage with Thermodynamics objective questions to reinforce their comprehension of concepts like entropy, enthalpy, and thermodynamic systems. These questions not only test theoretical knowledge but also practical applications, challenging learners to apply thermodynamic principles to real-world scenarios. A Thermodynamics quiz typically includes Thermodynamics multiple choice questions designed to cover these fundamental concepts comprehensively. Such quizzes are valuable for self-assessment and preparation for exams, ensuring a thorough grasp of Thermodynamics principles across various contexts, from engineering to environmental sciences. |
Thermodynamics Online Quiz
By presenting 3 options to choose from, Thermodynamics Quiz which cover a wide range of topics and levels of difficulty, making them adaptable to various learning objectives and preferences. You will have to read all the given answers of Thermodynamics Questions and Answers and click over the correct answer.
- Test Name: Thermodynamics MCQ Quiz Practice
- Type: Quiz Test
- Total Questions: 40
- Total Marks: 40
- Time: 40 minutes
Note: Answer of the questions will change randomly each time you start the test. Practice each quiz test at least 3 times if you want to secure High Marks. Once you are finished, click the View Results button. If any answer looks wrong to you in Quiz, simply click on question and comment below that question, so that we can update the answer in the quiz section.
Download Certificate of Thermodynamics Test
On the end of Quiz, you can download the certificate of the quiz if you got more than 70% marks.
Thermodynamics Flashcards
What is the branch of physical science that deals with the relationships between heat and other forms of energy?
Thermodynamics
Which law of thermodynamics states that energy cannot be created or destroyed, only converted from one form to another?
First law of thermodynamics
What is the equation representing the first law of thermodynamics for a closed system undergoing a process?
ΔU=Q−WDelta U = Q - WΔU=Q−W
What is the term for the heat transferred between a system and its surroundings at constant volume?
Heat capacity
What is the term for the heat transferred between a system and its surroundings at constant pressure?
Enthalpy change
What is the formula for calculating the change in internal energy (ΔUDelta UΔU) of a system?
ΔU=Q+WDelta U = Q + WΔU=Q+W
What is the formula for calculating the change in enthalpy (ΔHDelta HΔH) of a system?
ΔH=Q+WDelta H = Q + WΔH=Q+W
What is the formula for calculating the change in entropy (ΔSDelta SΔS) of a system?
ΔS=Q/TDelta S = Q / TΔS=Q/T
What is the term for a process that occurs without the exchange of heat with the surroundings?
Adiabatic
What is the term for the maximum amount of work that can be done by a system on its surroundings?
Gibbs free energy
What is the term for a spontaneous process that occurs without the input of external energy?
Exothermic
What is the term for the study of the relationships between heat, work, and energy in a chemical or physical process?
Thermochemistry
What is the term for the change in Gibbs free energy of a system at constant temperature and pressure?
ΔGDelta GΔG
What is the formula for calculating Gibbs free energy (GGG) from enthalpy (HHH) and entropy (SSS)?
G=H−TSG = H - TSG=H−TS
What is the term for the change in Helmholtz free energy of a system at constant temperature and volume?
ΔADelta AΔA
What is the formula for calculating Helmholtz free energy (AAA) from internal energy (UUU) and entropy (SSS)?
A=U−TSA = U - TSA=U−TS
What is the term for the change in Gibbs free energy of a system at constant temperature?
ΔGDelta GΔG
If you are interested to enhance your knowledge regarding Physics, Computer, and Biology please click on the link of each category, you will be redirected to dedicated website for each category.